100 years ago, physicist Wolfgang Pauli published his exclusion principle, a foundational concept that impacted our understanding of basic chemistry, revealed more about the structure of the atom, and even explained the behaviour of neutron stars. The concept is so important that physicists use it to classify subatomic particles: those that follow the exclusion principle are called fermions, and those that don’t are called bosons.
Without the exclusion principle, scientists would have no understanding as to why stable molecules exist or how electrons arrange themselves in atoms. But what is it exactly, and why is it so foundational?
Pauli’s exclusion principle, explained
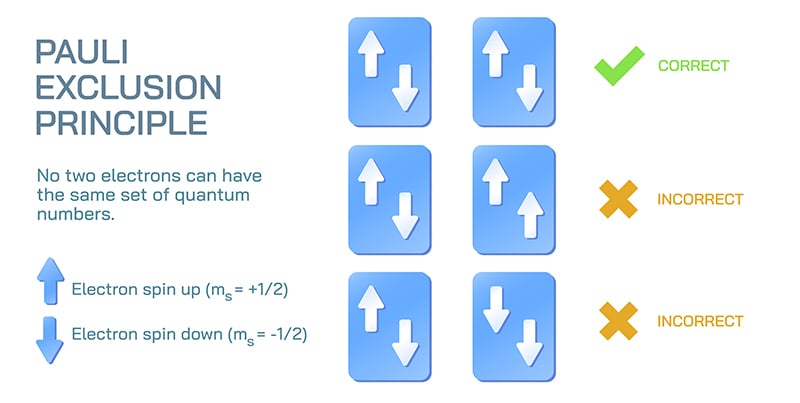
At its heart, the exclusion principle is simple: no two identical fermions in a system can occupy the same quantum state. In a standard atom, for example, the exclusion principle dictates that every electron in that atom cannot occupy the same quantum state. And what is a quantum state? “The state is the collection of all the things you need to specify the particle,” says Leonardo A. Lessa, a PhD student currently studying quantum matter at Perimeter Institute. “For the Standard Model of physics, it’s a combination of where the particles are in real space and some internal degrees of freedom.”
In the case of a single electron in an atom, that combination of real space and internal degrees of freedom are summed up in four numbers called the quantum numbers. The numbers essentially outline a particle’s distance from the nucleus, describe its orbit in shape and energy, and assign it a spin value. The spin quantum number is particularly important in Pauli’s theory because he developed it to help explain his principle. Although, as Lessa point out, it’s not what it seems.
“Spin is like as if the particle was a ball that’s spinning. But it’s not a ball and it’s not spinning,” he says, quoting a joke common amongst quantum researchers. “But of course it has its name because it acts like a spin. It physically gives the particle angular momentum. Because if things are spinning, they have angular momentum on some axis. Spin is a kind of intrinsic angular momentum that the particle can have.”
And that’s the exclusion principle: when you describe identical fermions in a system, none of them can have identical values in all four quantum numbers.
So how did Pauli discover this fundamental concept and, in the process, create a totally new quantum number? It’s a journey that saw him interacting with some of the greatest physicists of his time.
Pauli’s journey to exclusion
A bit of a prodigy, Pauli was born in 1900, the same year that Max Planck published his idea of quanta energy that kicked off quantum mechanics. He grew up fascinated with these leaps forward, reading Einstein in grade school and pursuing theoretical physics in university.
Pauli was also notorious for two reasons. First were his scathing critiques of ideas he thought underdeveloped. He once famously described a paper as so poor that it “wasn’t even wrong,” a label used to this day for poor and pseudoscientific theories. Second was the Pauli Effect, a tendency for experimental equipment to break down whenever he was near. While the latter was never peer reviewed, Pauli did reportedly take delight whenever equipment would coincidentally fail when he was in a lab.
Pauli was initially skeptical of quantum theory, something he described as a “shock which every physicist... experienced” in his lecture for winning the Nobel Prize in Physics. During his studies at the University of Munich, he was introduced to the structure of the atom by Arnold Sommerfeld, his PhD supervisor and an important figure in quantum mechanics. Prominent physicists at the time were trying to reconcile classical mechanics with the new quantum approach to serve as a key to understanding both. At the time, the series of numbers made with 2n2 (2, 8, 18, 32, for example) were known to be critical to understanding chemistry, but they didn’t know why.
Things changed in 1922, when Pauli met Niels Bohr, the physicist who developed a model of the atom that accounted for quantum mechanics. It was during this time that Pauli was introduced to electron shells: the idea that electrons surround the nucleus in a series of orbits increasingly far away from the centre. Each shell has a different energy level based on its distance, and every element has a certain number of shells based in part on how many electrons they have. In fact, we arrange the rows of the periodic table of the elements based on their number of shells.
Electron shells made sense, but Pauli and others couldn’t understand why all electrons didn’t simply end up in the shell closest to the nucleus, in the lowest energy state. After all, most systems eventually arrive at a place of lowest energy, so why weren’t the subatomic particles doing this consistently, too?
Something was missing, something that could help further describe the quantum state of each particle. In his research, Pauli landed on something he called “two-valuedness not describable classically.” That became the spin, the fourth quantum number. The ball that spins without being a ball or spinning. The two-valuedness aspect stems from whether the particle has an upward spin, referred to as “spin up,” or a downward spin, otherwise known as “spin down.”
With the fourth quantum number, Pauli could now explain how electrons can exist in multiple energy states around a nucleus: because electrons can’t hold the same value in all four quantum numbers. Instead, each shell’s electrons must differ slightly. It also meant that each shell moving outward could at maximum include 2, 8, 18, 32 electrons, and so on.
Pauli’s idea in the real world (and in the universe)
Today, the exclusion principle helps us not only understand tiny things like electrons but also massive objects like neutron stars. In fact, it’s the exclusion principle that stops neutron stars from turning into black holes.
“Neutron stars are a type of celestial body that form under very specific extreme conditions in the universe,” explains Lessa. “It’s a very compact object made almost exclusively of neutrons.”
Classically, stars have a balance of forces pushing inward and outward. Gravity pushes a star together while its nuclear reactions push outward. When gravity exceeds the outward pressure of the nuclear reactions, stars start to collapse.
Neutron stars occur when supermassive stars collapse, which is one way that black holes can form. Except in a neutron star, the gravity becomes so intense that the electrons and protons in atoms undergo a reaction that crush them into neutrons.
Once the neutrons form, they pack together much more tightly because there’s no charge pushing them apart. They then start to exert a different force called “neutron degeneracy pressure.” Along with the repulsive nuclear force between neutrons, this prevents further collapse due to the exclusion principle, which restricts fermions like neutrons from occupying the same quantum state. The neutrons stay far enough apart that they can’t cross the threshold for turning into a black hole, unless something else is added, like additional matter or if the star was simply too large to begin with.
With the exclusion principle, physicists at Perimeter and beyond continue to make discoveries related to the smallest and largest parts of our universe. From understanding chemical reactions to the formation of black holes, Pauli’s principle lays the groundwork for future discovery.
“It’s beautiful,” says Lessa, “that at some point you could reach an understanding from fundamental principles to explain why things are how they are. Even though these rules of quantum mechanics are so fragile, in a sense, they conspire to make things work as they should.”
About PI
Perimeter Institute is the world’s largest research hub devoted to theoretical physics. The independent Institute was founded in 1999 to foster breakthroughs in the fundamental understanding of our universe, from the smallest particles to the entire cosmos. Research at Perimeter is motivated by the understanding that fundamental science advances human knowledge and catalyzes innovation, and that today’s theoretical physics is tomorrow’s technology. Located in the Region of Waterloo, the not-for-profit Institute is a unique public-private endeavour, including the Governments of Ontario and Canada, that enables cutting-edge research, trains the next generation of scientific pioneers, and shares the power of physics through award-winning educational outreach and public engagement.